Dr. Haneesha Mohan is a Postdoctoral Research Fellow at the University Health Network and has both a Canadian Institutes of Health Research (CIHR) Fellowship Award and a CIHR Canadian HIV Trials Network (CTN) Postdoctoral Fellowship Award. She is supervised by Dr. Lena Serghides. Haneesha earned her Bachelor of Life Science (Hons.) degree in Life Sciences with a Minor in Health Geography at McMaster University, and then pursued her PhD at the University of Saskatchewan in Integrative Neuroendocrinology.
HIV was once a fatal disease. However, through scientific discovery and drug development, HIV has become a chronic manageable disease. While the health benefits of HIV antiretrovirals (ARVs) are undeniable, ARV medications can have side effects. For example, ARVs have been implicated in dysregulation of glucose metabolism. I have always been keen to determine how novel pharmacologic approaches in the field of HIV affect whole body energy metabolism.
Dolutegravir (DTG), which is part of World Health Organization recommended first-line regimens, has been associated with hyperglycemia and serum aminotransferase elevations. DTG has also been associated with increased risk for neural tube defects (NTD). It is not yet known how DTG causes these metabolic perturbations and adverse birth outcomes.
Why this research?
DTG is metabolized by the liver, largely by glucuronidation with subsequent urinary clearance. This intrigued me and I chose to use my background in liver and islet metabolism to study the effects of DTG on glucose and energy metabolism. I wanted to provide a mechanistic understanding and evaluate the long-term implications of epidemiological observations of ARV-associated hyperglycemia in individuals living with HIV. As abdominal fat gain, weight gain, and rising body mass index (BMI) during HIV treatment increase the incident diabetes risk, I wanted to transpose my experience in molecular and cellular biology towards the understanding of metabolic diseases and birth defects caused by ARV medications.
ARV medications can cause hormonal changes that may affect fetal and maternal metabolism. While ARV medications are very good at preventing transmission of HIV from mother to child, the secondary effects, which include metabolic perturbations and negative effects on early fetal development, remain a concern. Many ARV medications have been associated with lipoatrophy and earlier onset diabetes, but longer-term data are lacking on the metabolic consequences of more modern regimens. Side effects of ARV medications include hyperglycemia, which may predispose women to NTD by altering metabolic homeostasis.
Recently, an ongoing NIH-funded observational study in Botswana revealed a higher incidence of NTD in women living with HIV who received DTG from conception. These findings highlighted the urgent need to improve our understanding of the underlying pathophysiology associated with DTG exposure in pregnancy and its associated metabolic complications. However, more studies are required to investigate the effects of DTG with respect to the duration of treatment, timing of drug exposure, and maternal nutritional status. My curiosity led me to join the Serghides lab, where I could explore this area and determine the effects of ARV medications on fetal development, maternal health, and glucose metabolism.
What does this mean on a global scale?
While the burden of adverse birth outcomes is highest in low resource settings, in part due to limitations in medical care resources, the rates of these adverse birth outcomes are rising around the world, making this a truly global health issue. The current lack of diagnostic tools to predict at-risk pregnancies, coupled with a lack of therapeutic intervention strategies, is a result of our poor understanding of the pathophysiology leading to adverse birth outcomes. In addition, future observational studies reporting the impact of diabetes and gestational diabetes in treated HIV infection are much awaited.
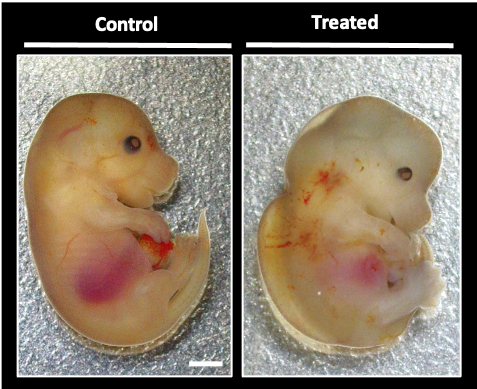
Using a mouse pregnancy model, I aim to understand the pathophysiologic mechanisms that underlie ARV-associated adverse birth defects, especially as they relate to metabolic alterations. Our results will improve our understanding of the toxicological effects of DTG use in pregnancy on fetal development. I am also keenly interested in exploring how maternal nutritional status and metabolic health contribute to fetal development and birth outcomes in the context of ARV use.
Although mice are not humans, rodent studies are advantageous as their fetal development period is much shorter than humans, and they circumvent the methodological limitations of human studies.
What does the future of this research look like?
This study will provide much needed evidence on whether DTG poses a risk for impaired glucose metabolism, NTD, and other congenital anomalies using an animal model. Our findings will address critical knowledge gaps in our understanding of the pathobiology of DTG-associated NTD and impaired metabolism. Our findings will help validate and interpret epidemiological findings and help direct future clinical studies.
One translational potential of our work is to develop antenatal diagnostic tests to predict risk for adverse fetal outcomes, so pregnant women at risk could be identified early and receive additional medical care to ensure best outcomes. Additionally, it is still a wide open question as to what is the best ARV regimen to use in pregnancy to ensure best outcomes for both mom and baby.